Molecular mechanisms of airway defence
Dr Vito Mennella
We are understanding how the airways respond, adapt and survive when confronted with different types of insults such as toxic particulates, drugs and infectious agents that target the airways.
Through breathing, from the early stages of human development throughout our adult life, we expose our lung to potentially harmful environmental pollutants, bacteria and viruses. From the nostrils to the trachea, to the deeper areas of our bronchi, the airway epithelium is there to protect our health throughout the life course.
The recent, rapid emergence of global health threats, such as COVID-19, has dramatically emphasized the key role that the airway epithelium plays in human health. Other less obvious, but significant threats have been affecting us since the industrial revolution such as environmental pollution. The most recent systematic analysis of the global burden of disease (Lancet, 2019), which includes data from more than 200 nations, has shown that airway exposure to pollution from ambient particulate matter (PM) is linked to a significant increase in risks of respiratory and cardiovascular disease.
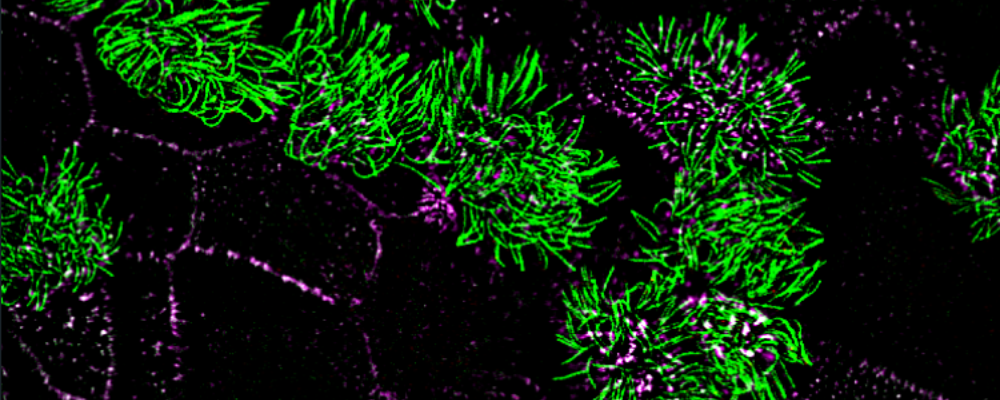
Airway cells have evolved clever molecular mechanisms protecting our tissues, which include physical trapping through molecular glues, mechanical removal by beating nanomachines, and rapid communication to mobilize innate and adaptive immune response. Our program investigates how these molecular mechanisms function in normal states and how they adapt or fail in the presence of different types of insults such as toxic particulates, drugs targeting the airways and infectious agents to identify biomarkers of disease and toxic states and rescue strategies.
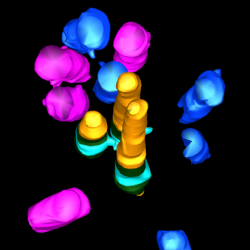
Toward this goal, we employ airway primary cellular models from different lung compartments and an airway-specific molecular toolbox to systematically identify key molecular events, common or different, in the adverse outcome pathways driven by potential toxicants. We leverage a range of molecular tools including CRISPR-Cas9 loss of function studies, proximity mapping protein-protein interaction analysis, and advanced imaging technologies such as super-resolution microscopy and volumetric EM to provide both a global and detailed view of airway cellular phenotypes in healthy and toxic states.
Key publications
Blume C, Jackson C, Spalluto M, Legebeke J, Nazlamova L, Conforti F, Perotin-Collard JM, Frank M, Crispin M, Coles J, Ridley R, Dean LSN, Loxham M, Azim A, Tariq K, Johnston S, Skipp PJ, Djukanovic R, Baralle D, McCormick C, Davies ED, Lucas J, Wheway G and Mennella V. A novel isoform of ACE-2 is expressed in human and bronchial respiratory epithelia and is upregulated in response to RNA respiratory virus infection. Nature Genetics; 53, 205-214. (2021).
Liu Z, Nguyen QPH, Nanjundappa R, Megherbi A, Delgehyr N, Doherty R, Thompson J, Jackson C, Dell S, Czymmek K, Munier A, Mahjoub M and Mennella V. Super-resolution reveals novel cilium in airway multiciliated cells. Developmental Cell; 55(2), 224-236. (2020).
Nguyen QPH, Liu Z, Zlock L, Coyaud E, Laurent E, Ouyan H, Finkbeiner W, Moraes T, Raught B and Mennella V. Comparative super-resolution map of basal foot in primary and motile cilia. Developmental Cell; 55(2), 209-223. (2020).
Liu Z, Nguyen QPH, Guan Q, Albulescu A, Erdman L, Mahdaviyeh Y, Kang J, Hong O, Hegele R, Moraes T, Dell S and Mennella V. Quantitative super-resolution toolbox for diagnosis of motile ciliopathies. Science Translational Medicine; 12(535)eaay0071. (2020).
Sydor A, Coyaud E, Rovelli C, Liu H, Laurent E, Raught B and Mennella V. PPP1R35 is a novel centrosomal protein that regulates centriole length in concert with microcephaly protein RTTN. eLife; 7:e37846. (2018).
Costain G, Liu Z, Mennella V, Radicioni G, Goczi AN, Albulescu A, Walker S, Ngan B, Manson D, Vali R, Khan M, Palaniyar N, Hill DB, Hall DA, Marshall CR, Knowles M, Zariwala MA, Kesimer M and Dell SD. Hereditary mucin deficiency cause by biallelic loss of function of MUC5B. Am J Respir Crit Care Med; 205(7), 761-768. (2022).