Submitted by Dr A. Srivastava on Tue, 23/01/2024 - 16:11
We all go through times when we are not on track, feeling a little blue and out of our usual form. These are the times when we need a little warmth from those close to us – some extra support, a helping hand. The same goes for the proteins in our cells.
Proteins are the workhorses in our cells and have numerous tasks to complete every day just to keep everything functioning. But, just like us, they are subjected to various daily stresses with tasks they simply must get done. So, it’s no wonder that our cells have a very special group of proteins, called chaperones, that lend a helping hand to these out-of-shape and stressed-out proteins. The term ‘chaperone’ originated in medieval France and was used for a responsible adult who accompanies and supervises young people. Similarly in cells, chaperone proteins ‘protect young proteins’. One example is keeping the proteins in shape so they can do their jobs. Classically, chaperones are known to be in close physical contact with proteins, helping them stay in the correct shape and structure.
However, researchers from the Sawarkar lab, along with their collaborators, have discovered that chaperones also bind to RNA – another important molecule found in cells.
“We already knew that chaperones were implicated in many cellular processes that involved RNA, but it did come as a surprise that chaperones had this ability to bind RNA molecules, as well as proteins. Once we had gained this knowledge, it opened up the question of why chaperones needed to bind to RNA when they already have enough to do helping proteins. What purpose does this RNA binding have for the cell?” shared Dr Sergio Leone, one of the lead authors on the study.
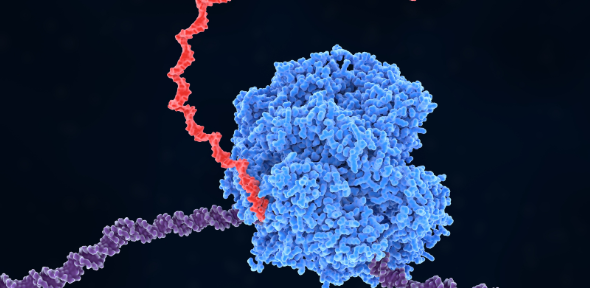
To explore this new question and understand why chaperones might interact with RNA, the researchers selected one chaperone and one class of RNA molecule. They chose a chaperone protein called HSP70, as it is one of the cell’s first responders when stressed, and transfer RNAs (tRNAs) – a group of so-called non-coding RNA which play an essential role in stringing proteins together from their building blocks called amino acids.
Transcription is a process in which protein complexes, called RNA polymerases, travel along the DNA, creating an RNA molecule using the DNA sequence as the template. There are three different RNA polymerases, but it is the RNA polymerase III (Pol III) that is responsible for transcribing the tRNA genes.
Playing detectives, the researchers found that HSP70 first binds to the Pol III, but subsequently, it jumps to the tRNA molecule that the Pol III is generating. It was already known that newly synthesised tRNAs can decrease the activity of Pol III leading to reduced production of tRNA, so by binding to the freshly generated tRNA, HSP70 helps to overcome this issue so that Pol III can continue to produce tRNA molecules.
The intricate molecular biology at play here is exciting because of the possibilities that arise are beyond the ‘detective’ work in the lab. tRNAs are essential for cell growth and proliferation. In fact, cancer cells need far more of them than normal cells. HSP70 inhibitors are already being studied as chemotherapeutic agents against several cancers. Here, the researchers have shown that HSP70 helps Pol III to produce tRNAs. To see if these findings have any potential in developing better combinations of chemotherapeutics, they analysed the publicly available cancer genomics data and did a few experiments themselves to show that inhibitors against HSP70 and Pol III, if used in combination, show a very potent effect on cancer cell death than what is pharmacologically expected, which could pave the way to more effective cancer therapies.
‘HSP70 binds to specific non-coding RNA and regulates human RNA polymerase III’ was published in Molecular Cell on 23rd January 2024. Read the full publication here.
Article by Dr Avinash Srivastava & Dr Sophie Milbourne

